
/Precision_PLUS_IPG_940x940-5a22ba107bb2830019b777b1.png)
The median (IQR) patient age was 60 (51-69) years 305 802 patients (55.3%) were female. Results Among 552 937 eligible patients treated between December 2015 and May 2021, 26 179 with PLS received an SCS implant. Opioid-naive patients were defined as those receiving at most 2 opioid prescriptions per year, and patients on LOT were those receiving at least 6 opioid prescriptions per year. Main Outcomes and Measures The main outcome was cessation of opioid use among patients receiving LOT or abstinence from opioids among opioid-naive patients. Statistical analysis was performed from June to December 2021. Patients were stratified according to baseline opioid use (opioid-naive or receiving LOT) and subsequent opioid therapy over the 12-month period ranging from 3 to 15 months post-SCS implantation or post-PLS index date. Objective To determine the association between SCS and long-term opioid therapy (LOT) for PLS.ĭesign, Setting, and Participants In this cohort study, adults with PLS were identified using the TriNetx Diamond Network and separated based on whether they underwent SCS. This has led to an increased emphasis on objective outcome measures such as opioid prescribing.

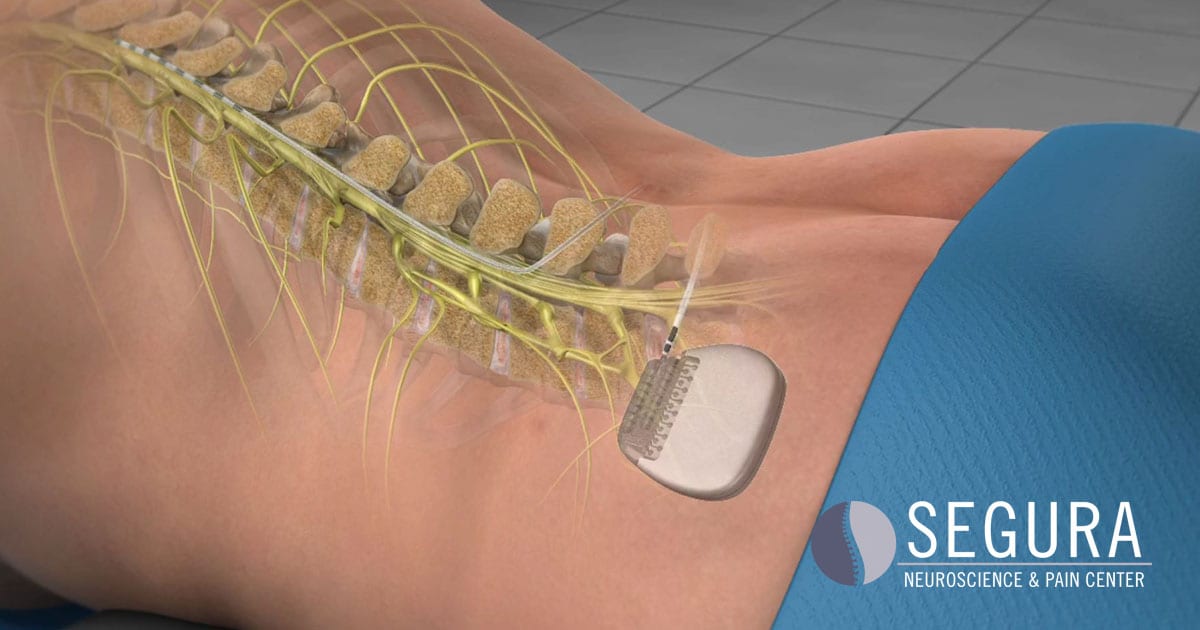
Importance The results of studies evaluating spinal cord stimulation (SCS) for postlaminectomy syndrome (PLS) have yielded mixed results. Shared Decision Making and Communication.Scientific Discovery and the Future of Medicine.Health Care Economics, Insurance, Payment.Clinical Implications of Basic Neuroscience.Challenges in Clinical Electrocardiography.Multivariable Analysis With Sensitivity Analyses Showing Factors Associated With New COT in Opioid-Naive Patients (Left Panel) and Persistent COT in Chronic Opioid Users (Right Panel) 3-15 Months After Adjusted Index Date (for Patients Without SCS) or SCS Implant in Diamond Network Breakdown of PLS Cohort by SCS Status, Baseline Opioid Status, and Opioid Use Status at 3-15 Months After Adjusted Index Date (for Patients Without SCS) or SCS Implant in Diamond NetworkĮFigure 4. Multivariable Analysis Showing Factors Associated With Receiving an SCS Implant in PLS PatientsĮFigure 3. Flowchart Illustrating the Inclusion and Exclusion Criteria of Postlaminectomy Syndrome Patients With and Without Spinal SCSĮFigure 2. Propensity Score Matching Analysis With Average Treatment Effect (ATE) Defined by Odds RatiosĮFigure 1. Characteristics of Post-Laminectomy Syndrome Patients in Diamond Network Stratified by SCS StatusĮTable 5. List of ICD-9/10 Codes Used to Calculate Charlson Comorbidity Index ScoreĮTable 4. List of Opioid and Psychotropic Medications Used With RxNorm Codes Included in ParenthesesĮTable 3. A List of ICD and CPT Codes Used to Extract Post-Laminectomy Syndrome Diagnosis, Spine Surgery Procedure, Spinal Cord Stimulation Device, and Other Relevant Clinical DiagnosesĮTable 2.


 0 kommentar(er)
0 kommentar(er)
